Global CAR T-Cell Therapy Market Forecast
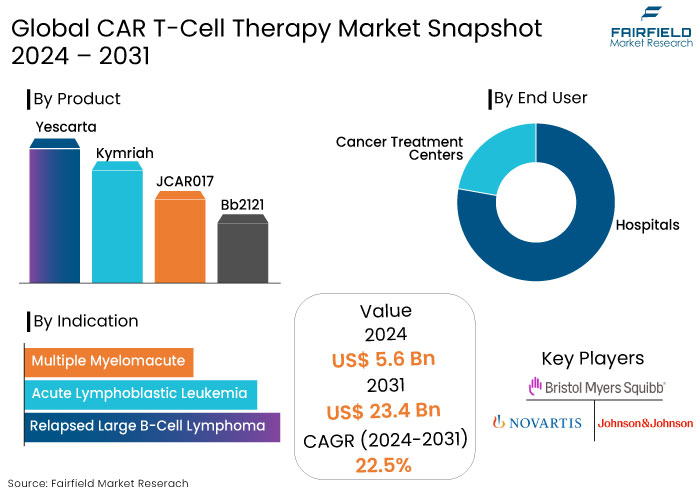
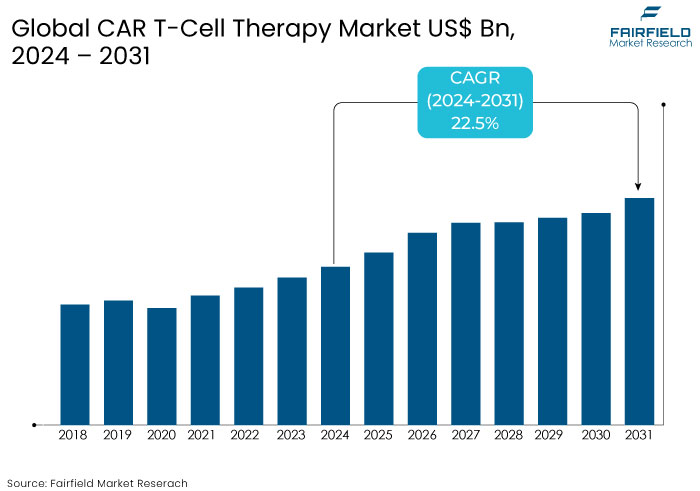
- The CAR T-Cell therapy market is projected to reach a size of US$23.4 Bn by 2031, showing significant growth from the US$5.6 Bn achieved in 2024.
- The market for CAR T-cell therapy is expected to show a significant expansion rate, with a healthy CAGR of 22.5% from 2024 to 2031.
CAR T-Cell Therapy Market Insights
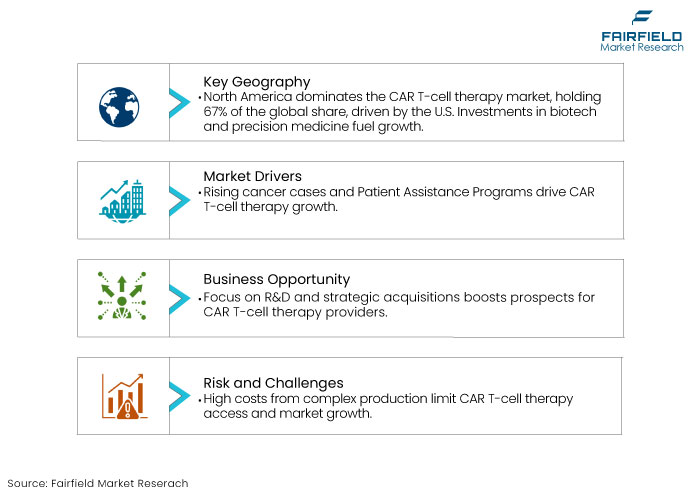
- North America dominates the market, with the U.S. accounting for the largest share due to advanced healthcare infrastructure and regulatory support.
- CAR T-cell therapies are primarily used for blood cancers, with high efficacy in treating leukemia, lymphoma, and multiple myeloma.
- Developing allogeneic (off-the-shelf) CAR T-cell therapies is seen as a critical innovation for reducing costs and improving scalability.
- Accelerated approvals and priority pathways fuel market expansion in regions like the North America and Europe.
- Partnerships between biopharma companies and research institutes are crucial for advancing CAR T-cell technology.
- Yescarta dominates the product segmentation with its robust performance and efficacy.
- Increasing investments in relapsed large b-cell lymphoma to demonstrate promise for cancer therapy.
- Novartis, Gilead Sciences, and Bristol-Myers Squibb lead the market, with biotech startups driving pipeline diversification.
A Look Back and a Look Forward - Comparative Analysis
The CAR T-cell therapy market experienced exponential growth during the period from 2019 to 2023 driven by advancements in immunotherapy and successful regulatory approvals. Landmark therapies such as Novartis' Kymriah and Gilead's Yescarta transformed cancer treatment, particularly for hematologic malignancies like leukemia and lymphoma.
North America dominated the market due to its advanced healthcare infrastructure and significant investments in biotechnology. Europe also witnessed rapid adoption following EMA approvals. However, the market faced challenges such as high treatment costs, manufacturing complexity, and adverse effect management, which limited its scalability.
The market is projected to enter a new growth phase over the forecast period, with anticipated advancements in "off-the-shelf" allogeneic CAR T-cell therapies, improving accessibility and reducing costs. Expansion into treating solid tumors is expected to diversify applications and drive demand.
Emerging markets in Asia Pacific are poised for substantial growth due to increasing healthcare investments and favorable regulatory frameworks. North America and Europe will continue leading the market but will face intensified competition from new entrants. Innovations in gene editing, automation in manufacturing, and improved safety profiles will catalyze broader adoption, pushing market growth further.
Key Growth Determinants
- Supportive Government Initiatives and Awareness Programs to Surge the Demand
The primary factor propelling the CAR T-cell treatment market is the rising incidence of cancer. The presence of Patient Assistance Programs (PAPs) is a contributing factor to the increased uptake of therapy.
Governments and groups are implementing efforts and programs to raise awareness about cancer, which also facilitates market expansion. Organizations such as the American Cancer Society and CancerCare provide an array of services designed to assist patients in managing the difficulties related to cancer treatment.
- According to recent research by the National Cancer Institute in the United States, cancer is the primary cause of death worldwide. Approximately 20 million new cancer cases were reported in 2022, with projections indicating an increase to 29.9 million by 2024.
The data indicates that the cancer rate in men exceeds that of women. The incidence of cancers such as prostate, lung, and colorectal cancer is projected to reach almost 48% in males by 2024. The data indicates the growing necessity for effective solutions such as CAR T-cell therapy and the role of government in fostering awareness of its significance.
- Increase in Clinical Trials to Establish New Pathways for Advanced Therapies
The proliferation of clinical studies is another key driver for the CAR T-cell therapy market growth. These trials seek to investigate novel applications and enhance current medicines. Pharmaceutical businesses and academic organizations are investing in developing sophisticated CAR T-cell therapies.
The huge investments are to target diverse malignancies and enhance patient outcomes. It encompasses experiments to improve the efficacy and safety profiles of CAR T-cells. Research examining combination therapies that incorporate CAR T-cell therapy with additional modalities.
The growing quantity of clinical trials substantiates the promise of CAR T-cell treatments, drawing investment and interest from stakeholders, which further accelerates market expansion.
Key Growth Barriers
- High Cost of Car T-Cell Therapy to Hamper Market Expansion
The exorbitant expense of CAR T-cell treatment constitutes a significant obstacle to its broad application and accessibility. The intricate and customized production process is the primary factor contributing to this exorbitant pricing. This factor hinders the CAR T-cell therapy market.
Each treatment involves the extraction and genetic modification of T-cells from the patient. This arduous and protracted process incurs substantial costs. Certain therapies, such as KYMRIAH and Yescarta, frequently exceed $350,000 per treatment.
The National Institute of Health data indicates that CAR T-cell therapy is the costliest treatment, with expenses ranging from $373,000 to over $500,000 per patient. The elevated cost is prompting global shifts in market acceptance.
- Stringent Eligibility Requirements for Therapy to Hinder Market Growth
Another hindrance for the CAR T-cell therapy market is its availability to patients who have previously received significant treatments. Patients must have undergone a minimum of two lines of systemic therapy to qualify for treatments such as tisagenlecleucel or axicabtagene ciloleucel.
Certain individuals who have undergone various therapies are apprehensive about enduring the severe adverse effects of chimeric antigen receptor T-cell treatments. Stringent eligibility requirements for CAR T-cell therapy correlate with elevated treatment expenses, which is expected to impede the uptake of CAR T treatment.
According to an article in MJH Life Sciences from April 2021, a recent examination of real-world data indicates that the average overall cost of CAR T-cell therapy surpasses US$700,000, with certain instances exceeding $1 million.
CAR T-Cell Therapy Market Trends and Opportunities
- Research and Development in Oncology Treatments to Generate Growth Opportunities
There is an increased emphasis on research and development for cancer medicines, including those for lung cancer, colorectal cancer, multiple myeloma, and prostate cancer. This trend is anticipated to generate numerous potential prospects for CAR T-cell treatment providers. Strategic acquisitions might assist manufacturers in expediting their endeavours to secure a greater market share.
Favourable government regulations and financial backing for life sciences are anticipated to stimulate demand for CAR T-cell therapy in the imminent future. The Life Sciences legislation designates US$1 billion over a decade to fund diverse programs in the United States.
The growing demand for novel cancer treatment alternatives is expected to generate substantial revenue prospects for participants in the chimeric immunoreceptor therapy industry.
- Progress in Therapeutics to Propel Industry Growth
The application of CAR T-cell therapy in solid tumours presents a significant prospect for the CAR T-cell therapy market expansion and medical breakthroughs. CAR T-cell treatments have demonstrated significant efficacy in the treatment of hematological malignancies such as B-cell lymphomas and Acute Lymphoblastic Leukemia (ALL). However, their application in solid tumours has proven to be more difficult.
It is mostly attributable to distinctive challenges, including the harsh tumour microenvironment, the heterogeneity of tumour cells, and the physical limitations present within solid tumours. Surmounting these challenges may result in novel chances for growth and innovation within the sector. Organizations in this sector are advancing many goods and pioneering therapies.
Stanford Medicine, located in the United States, has become the inaugural American institution to use a novel cell-based therapy to treat metastatic melanoma. It is the inaugural treatment sanctioned by the FDA for solid tumours. Like CAR-T therapies, this therapy uses immune cells to augment cancer-fighting capabilities.
How Does Regulatory Scenario Shape this Industry?
The regulatory landscape is pivotal in shaping the CAR T-cell therapy market, driving its growth and accessibility while ensuring patient safety. Key regions like North America, Europe, and Asia Pacific have developed frameworks to expedite the approval and commercialization of these transformative therapies.
The FDA has been at the forefront in the United States, granting accelerated approvals to landmark therapies like Kymriah (Novartis) and Yescarta (Gilead Sciences). The agency’s priority review and breakthrough therapy designations have streamlined the development process, fostering innovation.
The European Medicines Agency (EMA) has approved several therapies under its PRIME (Priority Medicines) scheme, boosting Europe market growth. Emerging economies in Asia Pacific, such as China and Japan, are revising regulatory guidelines to attract clinical trials and accelerate approvals.
China’s National Medical Products Administration (NMPA) introduced policies to support local CAR T-cell manufacturing, creating a competitive global market. Regulatory challenges persist, including the high costs of compliance, complex manufacturing standards, and addressing long-term safety concerns.
Regulators worldwide focus on refining guidelines for allogeneic (off-the-shelf) CAR T-cell therapies, aiming to expand accessibility and affordability. The evolving regulatory framework underpins market expansion, driving innovation and broad adoption globally.
Segments Covered in the Report
- Yescarta Dominates the Market with Its Robust Performance and Efficacy
Yescarta is projected to have a substantial market share of 57% in by 2024. Yescarta therapy is specifically intended for individuals with large B-cell lymphoma who have exhibited inadequate responses to conventional treatment modalities. Its robust market performance demonstrates its efficacy in meeting the demands of patients with this difficult disease.
A substantial randomized phase 3 clinical trial of CAR T-cell treatment Yescarta has demonstrated its potential to cure certain individuals with aggressive non-Hodgkin lymphoma (NHL). The research showcased at the American Society of Clinical Oncology (ASCO) annual meeting projected that 55% of patients would remain alive four years’ post-axis-cell treatment, in contrast to 46% of those who initially had standard therapy for relapsed disease.
The findings indicate that axis-cell is currently the preferred therapy for those with rapidly recurring or treatment-resistant diffuse large B-cell lymphoma.
- Increasing Investments in Relapsed Large B-Cell Lymphoma
Relapsed large B-cell lymphoma is projected to have approximately 90% of the market share in 2024. The substantial market share underscores the critical demand for efficacious medicines in addressing this difficult lymphoma, as patients frequently encounter restricted therapy alternatives upon recurrence or lack of improvement of their illness.
Diverse corporations and institutions are allocating resources to research and comprehend big B-cell lymphoma and its treatment. The Phase 3 ECHELON-3 research conducted by the American pharmaceutical and biotechnology corporation Pfizer showed that ADCETRIS, in conjunction with lenalidomide and rituximab, decreased the mortality risk of patients by 37% relative to placebo. The study findings indicate a novel optimism for cancer therapy.
Regional Analysis
- North America Remains Dominant with Huge Investment in Biotechnology & Healthcare Industry
North America holds approximately 67% of the global CAR T-cell therapy market share, with the United States contributes greatly toward growth. High levels of investment in biotechnology and healthcare industries ensure robust revenue growth. Established companies with a focus on precision medicine support the market.
The incidence of hematologic malignancies like leukemia, lymphoma, and multiple myeloma is high in the United States and Canada. The demand for targeting this prevalence drives effective therapies like CAR T-cell therapy.
North America benefits from a well-developed healthcare infrastructure capable of supporting advanced therapies. Access to specialized hospitals and treatment centers facilitates the implementation of CAR T-cell therapy.
The FDA has approved multiple CAR T-cell therapies, such as Novartis' Kymriah and Gilead's Yescarta, boosting market growth. Accelerated approval pathways and supportive clinical trial frameworks encourage innovation.
North America's dominance in market is underpinned by its advanced healthcare infrastructure, significant research and development investments, and a supportive regulatory environment. While challenges exist, the region is poised to maintain its leadership through innovation and expansion of treatment applications.
- Asia Pacific CAR T-Cell Therapy Market Emerges Lucrative with Robust Government Support
Asia Pacific is anticipated to experience substantial growth in CAR T-cell treatment market by 2031. This region is estimated to expand at a CAGR of 28% from 2024 to 2031. An increasing patient demographic and robust governmental backing for products and market stakeholders propels this swift growth.
Prominent players are progressively concentrating on the development of improved medicines, hence enhancing market growth. China's National Medical Products Administration (NMPA) has sanctioned the supplemental biological license application for JW Therapeutics' Carteyva, intended for people with relapsed or refractory mantle cell lymphoma. A clinical investigation in China additionally corroborates this supplement.
The rising number of clinical studies also facilitates industry growth. The Clinical Studies Database 2023 indicates that approximately 103 CAR T treatments are presently undergoing active clinical studies in China.
Fairfield’s Competitive Landscape Analysis
The CAR T-cell therapy market is intensely competitive, dominated by leading players like Novartis, Gilead Sciences (Kite Pharma), and Bristol-Myers Squibb, pioneers in commercializing landmark therapies such as Kymriah, Yescarta, and Breyanzi.
Emerging biotech companies, including Legend Biotech and CARsgen Therapeutics, are driving innovation with novel approaches to CAR T-cell engineering and solid tumor treatments. The market also witnesses regional players, particularly in Asia Pacific, leveraging cost-effective manufacturing to challenge global leaders. Partnerships between biotech firms and research institutions are fostering rapid advancements.
Key competitive factors include proprietary technology, manufacturing capabilities, regulatory approvals, and pipeline diversification. The race for allogeneic therapies and expanded indications is intensifying, creating opportunities for new entrants and driving market dynamics.
Key Market Companies
- Bristol-Myers Squibb Company
- Novartis AG
- Gilead Sciences, Inc.
- Johnson & Johnson Services, Inc.
- JW Therapeutics (Shanghai) Co., Ltd.
- Bluebird Bio, Inc.
- Merck & Co., Inc.
- Sangamo Therapeutics
- Sorrento Therapeutics, Inc.
- GSK PLC
Recent Industry Developments
- In April 2024, India introduced its inaugural indigenously developed CAR T-cell therapy for cancer treatment, marking a significant advancement in combating the disease. The gene therapy, created by IIT Bombay and Tata Memorial Centre, is being introduced in India at one-tenth of its cost outside.
- In September 2024, MOC Cancer Care and Research Centre in Mumbai commenced offering CAR-T Cell Therapy for patients with blood cancers, including Leukemias, Myeloma, and Lymphomas. The said therapy seeks to enhance results for individuals who have depleted standard treatment alternatives.
An Expert’s Eye
- Researchers are optimistic about ongoing advancements targeting solid tumours, though achieving similar success remains a significant challenge for CAR T-cell therapy market.
- Allogeneic or "off-the-shelf" CAR T-cell therapies are a key innovation to reduce costs and broaden accessibility.
- Collaborative efforts between regulatory agencies and biotech firms accelerate approvals and expand clinical trials globally.
- Experts highlight significant growth potential in Asia Pacific due to increasing healthcare investments and regulatory reforms.
Global CAR T-Cell Therapy Market is Segmented as-
By Product
- Yescarta
- Kymriah
- JCAR017
- Bb2121
By Indication
- Relapsed Large B-Cell Lymphoma
- Acute Lymphoblastic Leukemia (ALL)
- Multiple Myeloma
By End User
- Hospitals
- Cancer Treatment Centers
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- The Middle East & Africa
1. Executive Summary
1.1. Global CAR T-Cell Therapy Market Snapshot
1.2. Future Projections
1.3. Key Market Trends
1.4. Regional Snapshot, by Value, 2024
1.5. Analyst Recommendations
2. Market Overview
2.1. Market Definitions and Segmentations
2.2. Market Dynamics
2.2.1. Drivers
2.2.2. Restraints
2.2.3. Market Opportunities
2.3. Value Chain Analysis
2.4. Porter’s Five Forces Analysis
2.5. COVID-19 Impact Analysis
2.5.1. Supply
2.5.2. Demand
2.6. Impact of Ukraine-Russia Conflict
2.7. Economic Overview
2.7.1. World Economic Projections
2.8. PESTLE Analysis
3. Global CAR T-Cell Therapy Market Outlook, 2019 - 2031
3.1. Global CAR T-Cell Therapy Market Outlook, by Product, Value (US$ Mn), 2019 - 2031
3.1.1. Key Highlights
3.1.1.1. Yescarta (axicabtagene ciloleucel)
3.1.1.2. Kymriah (tisagenlecleucel)
3.1.1.3. JCAR017 (lisocabtagene maraleucel)
3.1.1.4. bb2121
3.2. Global CAR T-Cell Therapy Market Outlook, by Indication, Value (US$ Mn), 2019 - 2031
3.2.1. Key Highlights
3.2.1.1. Relapsed Large B-cell Lymphoma
3.2.1.2. Acute Lymphoblastic Leukemia (ALL)
3.2.1.3. Multiple Myeloma
3.3. Global CAR T-Cell Therapy Market Outlook, by End User, Value (US$ Mn), 2019 - 2031
3.3.1. Key Highlights
3.3.1.1. Hospitals
3.3.1.2. Cancer Treatment Centers
3.4. Global CAR T-Cell Therapy Market Outlook, by Region, Value (US$ Mn), 2019 - 2031
3.4.1. Key Highlights
3.4.1.1. North America
3.4.1.2. Europe
3.4.1.3. Asia Pacific
3.4.1.4. Latin America
3.4.1.5. Middle East & Africa
4. North America CAR T-Cell Therapy Market Outlook, 2019 - 2031
4.1. North America CAR T-Cell Therapy Market Outlook, by Product, Value (US$ Mn), 2019 - 2031
4.1.1. Key Highlights
4.1.1.1. Yescarta (axicabtagene ciloleucel)
4.1.1.2. Kymriah (tisagenlecleucel)
4.1.1.3. JCAR017 (lisocabtagene maraleucel)
4.1.1.4. bb2121
4.2. North America CAR T-Cell Therapy Market Outlook, by Indication, Value (US$ Mn), 2019 - 2031
4.2.1. Key Highlights
4.2.1.1. Relapsed Large B-cell Lymphoma
4.2.1.2. Acute Lymphoblastic Leukemia (ALL)
4.2.1.3. Multiple Myeloma
4.3. North America CAR T-Cell Therapy Market Outlook, by End User, Value (US$ Mn), 2019 - 2031
4.3.1. Key Highlights
4.3.1.1. Hospitals
4.3.1.2. Cancer Treatment Centers
4.3.2. BPS Analysis/Market Attractiveness Analysis
4.4. North America CAR T-Cell Therapy Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
4.4.1. Key Highlights
4.4.1.1. U.S. CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
4.4.1.2. U.S. CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
4.4.1.3. U.S. CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
4.4.1.4. Canada CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
4.4.1.5. Canada CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
4.4.1.6. Canada CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
4.4.2. BPS Analysis/Market Attractiveness Analysis
5. Europe CAR T-Cell Therapy Market Outlook, 2019 - 2031
5.1. Europe CAR T-Cell Therapy Market Outlook, by Product, Value (US$ Mn), 2019 - 2031
5.1.1. Key Highlights
5.1.1.1. Yescarta (axicabtagene ciloleucel)
5.1.1.2. Kymriah (tisagenlecleucel)
5.1.1.3. JCAR017 (lisocabtagene maraleucel)
5.1.1.4. bb2121
5.2. Europe CAR T-Cell Therapy Market Outlook, by Indication, Value (US$ Mn), 2019 - 2031
5.2.1. Key Highlights
5.2.1.1. Relapsed Large B-cell Lymphoma
5.2.1.2. Acute Lymphoblastic Leukemia (ALL)
5.2.1.3. Multiple Myeloma
5.3. Europe CAR T-Cell Therapy Market Outlook, by End User, Value (US$ Mn), 2019 - 2031
5.3.1. Key Highlights
5.3.1.1. Hospitals
5.3.1.2. Cancer Treatment Centers
5.3.2. BPS Analysis/Market Attractiveness Analysis
5.4. Europe CAR T-Cell Therapy Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
5.4.1. Key Highlights
5.4.1.1. Germany CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.2. Germany CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.3. Germany CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.1.4. U.K. CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.5. U.K. CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.6. U.K. CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.1.7. France CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.8. France CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.9. France CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.1.10. Italy CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.11. Italy CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.12. Italy CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.1.13. Turkey CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.14. Turkey CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.15. Turkey CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.1.16. Russia CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.17. Russia CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.18. Russia CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.1.19. Rest of Europe CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
5.4.1.20. Rest of Europe CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
5.4.1.21. Rest of Europe CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
5.4.2. BPS Analysis/Market Attractiveness Analysis
6. Asia Pacific CAR T-Cell Therapy Market Outlook, 2019 - 2031
6.1. Asia Pacific CAR T-Cell Therapy Market Outlook, by Product, Value (US$ Mn), 2019 - 2031
6.1.1. Key Highlights
6.1.1.1. Yescarta (axicabtagene ciloleucel)
6.1.1.2. Kymriah (tisagenlecleucel)
6.1.1.3. JCAR017 (lisocabtagene maraleucel)
6.1.1.4. bb2121
6.2. Asia Pacific CAR T-Cell Therapy Market Outlook, by Indication, Value (US$ Mn), 2019 - 2031
6.2.1. Key Highlights
6.2.1.1. Relapsed Large B-cell Lymphoma
6.2.1.2. Acute Lymphoblastic Leukemia (ALL)
6.2.1.3. Multiple Myeloma
6.3. Asia Pacific CAR T-Cell Therapy Market Outlook, by End User, Value (US$ Mn), 2019 - 2031
6.3.1. Key Highlights
6.3.1.1. Hospitals
6.3.1.2. Cancer Treatment Centers
6.3.2. BPS Analysis/Market Attractiveness Analysis
6.4. Asia Pacific CAR T-Cell Therapy Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
6.4.1. Key Highlights
6.4.1.1. China CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
6.4.1.2. China CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
6.4.1.3. China CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
6.4.1.4. Japan CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
6.4.1.5. Japan CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
6.4.1.6. Japan CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
6.4.1.7. South Korea CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
6.4.1.8. South Korea CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
6.4.1.9. South Korea CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
6.4.1.10. India CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
6.4.1.11. India CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
6.4.1.12. India CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
6.4.1.13. Southeast Asia CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
6.4.1.14. Southeast Asia CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
6.4.1.15. Southeast Asia CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
6.4.1.16. Rest of Asia Pacific CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
6.4.1.17. Rest of Asia Pacific CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
6.4.1.18. Rest of Asia Pacific CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
6.4.2. BPS Analysis/Market Attractiveness Analysis
7. Latin America CAR T-Cell Therapy Market Outlook, 2019 - 2031
7.1. Latin America CAR T-Cell Therapy Market Outlook, by Product, Value (US$ Mn), 2019 - 2031
7.1.1. Key Highlights
7.1.1.1. Yescarta (axicabtagene ciloleucel)
7.1.1.2. Kymriah (tisagenlecleucel)
7.1.1.3. JCAR017 (lisocabtagene maraleucel)
7.1.1.4. bb2121
7.2. Latin America CAR T-Cell Therapy Market Outlook, by Indication, Value (US$ Mn), 2019 - 2031
7.2.1. Key Highlights
7.2.1.1. Relapsed Large B-cell Lymphoma
7.2.1.2. Acute Lymphoblastic Leukemia (ALL)
7.2.1.3. Multiple Myeloma
7.3. Latin America CAR T-Cell Therapy Market Outlook, by End User, Value (US$ Mn), 2019 - 2031
7.3.1. Key Highlights
7.3.1.1. Hospitals
7.3.1.2. Cancer Treatment Centers
7.3.2. BPS Analysis/Market Attractiveness Analysis
7.4. Latin America CAR T-Cell Therapy Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
7.4.1. Key Highlights
7.4.1.1. Brazil CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
7.4.1.2. Brazil CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
7.4.1.3. Brazil CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
7.4.1.4. Mexico CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
7.4.1.5. Mexico CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
7.4.1.6. Mexico CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
7.4.1.7. Argentina CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
7.4.1.8. Argentina CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
7.4.1.9. Argentina CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
7.4.1.10. Rest of Latin America CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
7.4.1.11. Rest of Latin America CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
7.4.1.12. Rest of Latin America CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
7.4.2. BPS Analysis/Market Attractiveness Analysis
8. Middle East & Africa CAR T-Cell Therapy Market Outlook, 2019 - 2031
8.1. Middle East & Africa CAR T-Cell Therapy Market Outlook, by Product, Value (US$ Mn), 2019 - 2031
8.1.1. Key Highlights
8.1.1.1. Yescarta (axicabtagene ciloleucel)
8.1.1.2. Kymriah (tisagenlecleucel)
8.1.1.3. JCAR017 (lisocabtagene maraleucel)
8.1.1.4. bb2121
8.2. Middle East & Africa CAR T-Cell Therapy Market Outlook, by Indication, Value (US$ Mn), 2019 - 2031
8.2.1. Key Highlights
8.2.1.1. Relapsed Large B-cell Lymphoma
8.2.1.2. Acute Lymphoblastic Leukemia (ALL)
8.2.1.3. Multiple Myeloma
8.3. Middle East & Africa CAR T-Cell Therapy Market Outlook, by End User, Value (US$ Mn), 2019 - 2031
8.3.1. Key Highlights
8.3.1.1. Hospitals
8.3.1.2. Cancer Treatment Centers
8.3.2. BPS Analysis/Market Attractiveness Analysis
8.4. Middle East & Africa CAR T-Cell Therapy Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
8.4.1. Key Highlights
8.4.1.1. GCC CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
8.4.1.2. GCC CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
8.4.1.3. GCC CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
8.4.1.4. South Africa CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
8.4.1.5. South Africa CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
8.4.1.6. South Africa CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
8.4.1.7. Egypt CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
8.4.1.8. Egypt CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
8.4.1.9. Egypt CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
8.4.1.10. Nigeria CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
8.4.1.11. Nigeria CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
8.4.1.12. Nigeria CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
8.4.1.13. Rest of Middle East & Africa CAR T-Cell Therapy Market by Product, Value (US$ Mn), 2019 - 2031
8.4.1.14. Rest of Middle East & Africa CAR T-Cell Therapy Market by Indication, Value (US$ Mn), 2019 - 2031
8.4.1.15. Rest of Middle East & Africa CAR T-Cell Therapy Market by End User, Value (US$ Mn), 2019 - 2031
8.4.2. BPS Analysis/Market Attractiveness Analysis
9. Competitive Landscape
9.1. By End User vs By Application Heat map
9.2. Manufacturer vs by Indication Heatmap
9.3. Company Market Share Analysis, 2024
9.4. Competitive Dashboard
9.5. Company Profiles
9.5.1. Novartis AG
9.5.1.1. Company Overview
9.5.1.2. Product Portfolio
9.5.1.3. Financial Overview
9.5.1.4. Business Strategies and Development
9.5.2. Gilead Sciences Inc.
9.5.2.1. Company Overview
9.5.2.2. Product Portfolio
9.5.2.3. Financial Overview
9.5.2.4. Business Strategies and Development
9.5.3. Celgene Corporation
9.5.3.1. Company Overview
9.5.3.2. Product Portfolio
9.5.3.3. Financial Overview
9.5.3.4. Business Strategies and Development
9.5.4. bluebird bio, Inc.
9.5.4.1. Company Overview
9.5.4.2. Product Portfolio
9.5.4.3. Financial Overview
9.5.4.4. Business Strategies and Development
9.5.5. Celyad Oncology
9.5.5.1. Company Overview
9.5.5.2. Product Portfolio
9.5.5.3. Financial Overview
9.5.5.4. Business Strategies and Development
9.5.6. Cartesian Therapeutics
9.5.6.1. Company Overview
9.5.6.2. Product Portfolio
9.5.6.3. Financial Overview
9.5.6.4. Business Strategies and Development
9.5.7. Intellia Therapeutics
9.5.7.1. Company Overview
9.5.7.2. Product Portfolio
9.5.7.3. Financial Overview
9.5.7.4. Business Strategies and Development
9.5.8. Sorrento Therapeutics, Inc
9.5.8.1. Company Overview
9.5.8.2. Product Portfolio
9.5.8.3. Financial Overview
9.5.8.4. Business Strategies and Development
10. Appendix
10.1. Research Methodology
10.2. Report Assumptions
10.3. Acronyms and Abbreviations
|
BASE YEAR |
HISTORICAL DATA |
FORECAST PERIOD |
UNITS |
|||
|
2023 |
|
2019 - 2023 |
2024 - 2031 |
Value: US$ Billion |
||
|
REPORT FEATURES |
DETAILS |
|
Product Coverage |
|
|
Indication Coverage |
|
|
End User Coverage |
|
|
Geographical Coverage |
|
|
Leading Companies |
|
|
Report Highlights |
Key Market Indicators, Macro-micro economic impact analysis, Technological Roadmap, Key Trends, Driver, Restraints, and Future Opportunities & Revenue Pockets, Porter’s 5 Forces Analysis, Historical Trend (2019-2021), Market Estimates and Forecast, Market Dynamics, Industry Trends, Competition Landscape, Category, Region, Country-wise Trends & Analysis, COVID-19 Impact Analysis (Demand and Supply Chain) |
