Global Triple Negative Breast Cancer Treatment Market Forecast
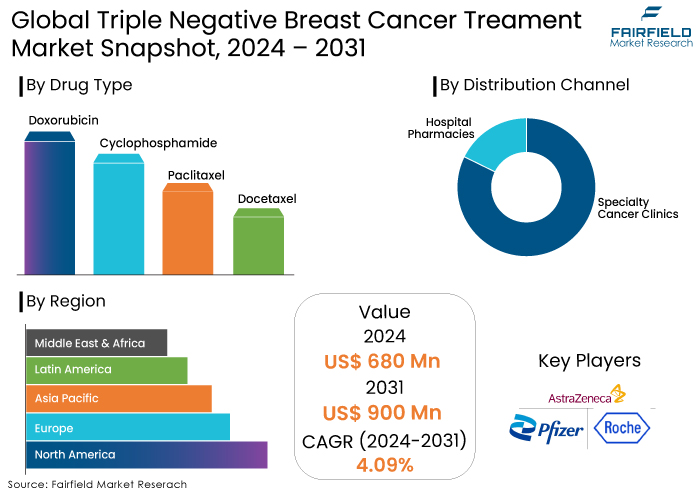
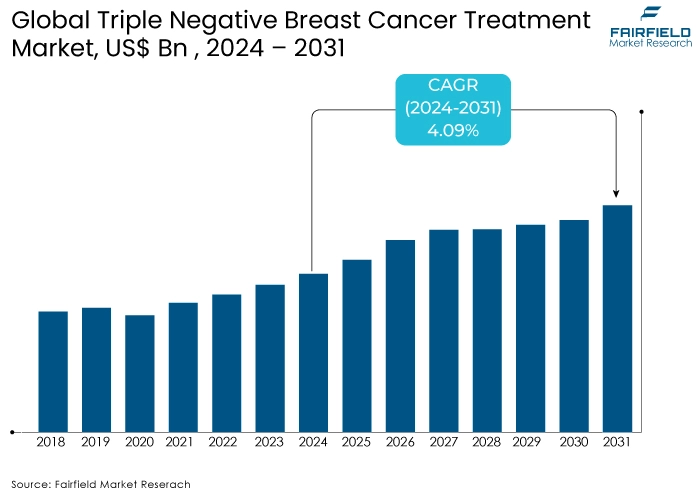
- Triple negative breast cancer treatment market size poised to reach US$900 Mn in 2031, up from US$680 Mn attained in 2024
- Global market triple negative breast cancer treatment is projected to witness a CAGR of 4.09% during 2024-2031
Triple Negative Breast Cancer Treatment Market Insights
- Triple negative breast cancer is characterized by the absence of clinically relevant levels of human epidermal growth factor receptor 2 (HER2), progesterone receptor (PR), and estrogen receptor (ER) expressions.
- This particular form of breast cancer has limited treatment options compared to other types of breast cancer and is a major contributor to the mortality rate among women.
- In addition, immune checkpoint inhibitors have had a substantial impact on the outcomes of medical treatments for patients, resulting in a fundamental change in the way triple negative malignancies are treated using programmed death ligand 1 (PD-L1).
- Breast cancer ranks as the second most widespread form of cancer and is the most often occurring cancer among women.
- Metastatic breast cancer is typically identified several months or even years following the diagnosis and treatment of early or advanced stages of breast cancer, such as stages 1, 2, or 3.
- Recent breakthroughs in cancer research have resulted in introducing more advanced and effective treatment alternatives, such as pharmaceutical therapy.
- The continuously advancing healthcare infrastructure and the rise in individuals' discretionary incomes are projected to drive market growth throughout the forecast period.
A Look Back and a Look Forward - Comparative Analysis
The triple negative breast cancer (TNBC) treatment market has experienced notable growth in the years leading up to 2023 and is poised for significant expansion post-2024. Pre-2023, the market's growth was driven by increasing incidence rates of TNBC, rising awareness about the disease, and advancements in diagnostic techniques. Key developments included approving targeted therapies and immunotherapies, such as immune checkpoint inhibitors, which provided new treatment options beyond traditional chemotherapy. Pharmaceutical companies invested heavily in research and development, leading to a robust pipeline of novel therapeutics to improve patient outcomes.
Collaborations and partnerships between biotech firms and research institutions further fueled innovation. The unmet medical need for effective TNBC treatments also spurred market growth, with healthcare providers and patients seeking better, more personalized treatment options.
Post-2024, the TNBC treatment market is expected to witness accelerated growth due to the anticipated approval of several promising drugs currently in late-stage clinical trials. Advances in precision medicine and the development of combination therapies are likely to play a significant role. Moreover, increasing investment in oncology research, favorable regulatory policies, and growing healthcare expenditure in emerging markets will contribute to market expansion. Enhanced diagnostic capabilities and the adoption of artificial intelligence in treatment planning are also expected to drive growth, offering more effective and tailored treatment solutions for TNBC patients.
Key Growth Determinants
- Advancements in Immunotherapy and Targeted Therapies
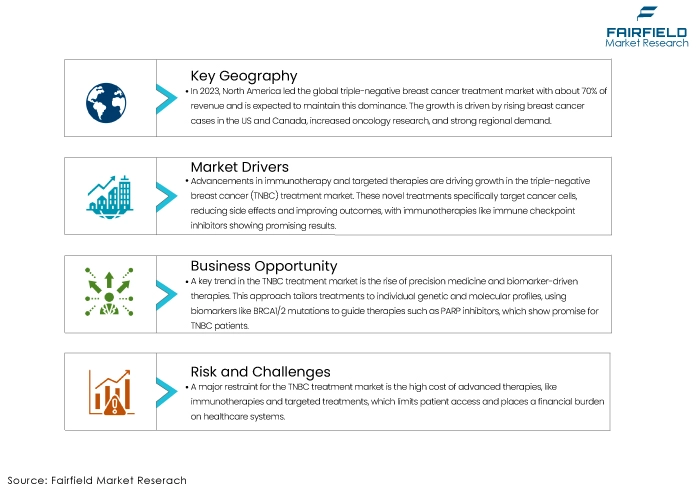
One of the primary growth drivers for the triple negative breast cancer (TNBC) treatment market is the advancement in immunotherapy and targeted therapies. Unlike traditional chemotherapy, which can affect cancerous and healthy cells, these novel therapies specifically target cancer cells, reducing side effects and improving patient outcomes. Immunotherapies, such as immune checkpoint inhibitors, have shown promising results in clinical trials, offering new hope for TNBC patients with limited treatment options.
Targeted therapies, including PARP inhibitors, are also gaining traction due to their ability to exploit specific genetic vulnerabilities in TNBC tumours.
The increasing adoption of these therapies, driven by ongoing research and positive clinical outcomes, is expected to boost the market significantly.
Additionally, pharmaceutical companies are heavily investing in developing new immunotherapeutic agents and combination therapies, further expanding the treatment landscape and providing more personalized and practical options for TNBC patients.
- Growing Incidence of Triple Negative Breast Cancer
The rising incidence of triple negative breast cancer is another crucial driver of market growth. TNBC accounts for approximately 10-20% of all breast cancer cases and is more prevalent among younger women, African American women, and those with BRCA1 mutations. This subtype of breast cancer is known for its aggressive nature and lack of targeted treatment options, leading to a higher demand for effective therapies.
As the global population ages and awareness about breast cancer screening increases, more cases of TNBC are being diagnosed. Early detection and diagnosis are crucial for effective treatment, and improved screening methods contribute to identifying more TNBC cases at earlier stages. This growing patient population is driving the demand for innovative treatments and encouraging pharmaceutical companies to focus on developing effective therapies. The heightened awareness and understanding of TNBC among healthcare professionals and patients also play a crucial role in driving market growth as they seek advanced treatment options to improve survival rates and quality of life.
Key Growth Barriers
- High Cost of Treatment and Limited Accessibility
One of the significant restraints for the triple negative breast cancer (TNBC) treatment market is the high cost associated with advanced therapies and limited accessibility. Treatments like immunotherapies and targeted therapies often come with substantial price tags, making them unaffordable for a large segment of patients, especially in low- and middle-income countries. The high cost limits patient access and places a significant financial burden on healthcare systems. Additionally, the availability of these advanced treatments is often restricted to specialized medical centres, which may not be accessible to patients in rural or underserved areas.
The healthcare infrastructure and insurance coverage disparity further exacerbate this issue, leading to unequal access to potentially life-saving treatments. Efforts to reduce costs through generic formulations, better insurance coverage, and expanded healthcare infrastructure are essential to overcoming this barrier and ensuring broader access to effective TNBC treatments.
- Challenges in Drug Development and Clinical Trials
Developing new treatments for TNBC faces several challenges that can restrain market growth. TNBC is a heterogeneous disease with diverse genetic and molecular profiles, making it challenging to develop one-size-fits-all treatments.
The complexity of the disease often leads to high failure rates in clinical trials, as promising therapies may need to show consistent efficacy across different patient subgroups.
Additionally, the stringent regulatory requirements for approving new drugs add to the time and cost of bringing new treatments to market. Recruiting sufficient patients for clinical trials is also challenging, particularly for a relatively rare subtype like TNBC. This can slow the progress of clinical research and ensure the availability of new treatments is maintained. Addressing these challenges requires innovative clinical trial designs, personalized medicine approaches, and collaborative efforts to streamline the drug development process and accelerate the approval of effective therapies for TNBC patients.
Triple Negative Breast Cancer Treatment Market Trends and Opportunities
- Adoption of Precision Medicine and Biomarker-Driven Therapies
One of the most significant trends in the triple negative breast cancer (TNBC) treatment market is the growing adoption of precision medicine and biomarker-driven therapies. Precision medicine involves tailoring treatments based on the genetic, molecular, and environmental factors specific to each patient's cancer. This approach is precious in TNBC, where traditional treatments have limited effectiveness. Researchers are increasingly focusing on identifying biomarkers to predict response to specific therapies. For example, BRCA1/2 mutations and other genomic alterations are being used to guide the use of PARP inhibitors, which have shown promise in treating TNBC patients with these mutations.
The integration of next-generation sequencing (NGS) and other advanced diagnostic tools into clinical practice is facilitating the identification of actionable biomarkers, enabling more personalized and effective treatment strategies. As a result, patients receive therapies that are more likely to be effective for their specific cancer subtype, improving outcomes and reducing unnecessary side effects. This trend is driving innovation in the pharmaceutical industry, with many companies investing in the development of targeted therapies and companion diagnostics.
Furthermore, precision medicine is encouraging more collaborative efforts between researchers, clinicians, and pharmaceutical companies, fostering a more holistic approach to cancer treatment. As this trend continues to evolve, it is expected to significantly enhance the management of TNBC, making treatments more effective and improving patient quality of life.
-
Opportunity Arising in Developing Regions
The TNBC treatment market is poised for significant growth in emerging markets, presenting a promising opportunity for expansion. Countries in Asia Pacific, Latin America, and the Middle East are experiencing increasing healthcare infrastructure development, rising disposable incomes, and improving access to medical care. These factors contribute to a growing demand for advanced cancer treatments, including TNBC treatments.
Emerging markets present a substantial opportunity for pharmaceutical companies to expand their footprint and tap into a large patient population with limited access to advanced therapies. Governments in these regions also recognize the importance of improving cancer care and investing in healthcare infrastructure, cancer awareness programs, and screening initiatives, which creates a conducive environment for introducing and adopting innovative TNBC treatments.
Furthermore, the growing prevalence of TNBC in these regions, driven by factors such as aging populations and changing lifestyle patterns, underscores the need for effective treatment options. Pharmaceutical companies can capitalize on this opportunity by partnering with local healthcare providers, conducting region-specific clinical trials, and tailoring their marketing strategies to meet the unique needs of these markets.
Efforts to make treatments more affordable through differential pricing, patient assistance programs, and collaborations with local governments are crucial in enhancing accessibility and driving market growth, fostering a sense of compassion and commitment to improving patient outcomes. By leveraging the potential of emerging markets, pharmaceutical companies can expand their market share and play a crucial role in addressing the global burden of TNBC, instilling a sense of empowerment and responsibility. This opportunity aligns with the broader goal of achieving equitable access to advanced cancer treatments and improving patient outcomes worldwide.
How is Regulatory Scenario Shaping this Industry?
The regulatory landscape is significantly influencing the market. Stringent regulatory requirements for drug approval, especially for cancer treatments, are shaping the industry. Regulatory bodies like the FDA and EMA are emphasizing the need for robust clinical trials demonstrating efficacy and safety. This has led to increased costs and timelines for drug development. However, these stringent regulations also ensure patient safety and drive innovation.
Additionally, regulatory policies around accelerated approval and breakthrough therapy designations are impacting the speed at which new treatments reach the market. While these pathways offer faster approval for promising drugs, they also come with post-marketing requirements to confirm benefits. Overall, the regulatory environment is a critical factor in the development and commercialization of TNBC therapies.
Segments Covered in Triple Negative Breast Cancer Treatment Market
- Paclitaxel-Loaded PLGA Nanofibers Provide Cost-Effective and Long-Lasting Drug Release
When it comes to pharmaceuticals, it is anticipated that the paclitaxel category will occupy 58% of the market share in the year 2024. Chemotherapy is the primary treatment for triple negative breast cancer that has spread to other parts of the body. Increasing the effectiveness of chemotherapy while avoiding systemic toxicity is possible using a method known as metronomic chemotherapy, which involves the delivery of chemotherapy in a continual low-dose manner. The idea of metronomic chemotherapy is congruent with the continuous and sustained drug release that is delivered by paclitaxel-loaded PLGA nanofibers. These nanofibers are more cost-effective for patients than chemotherapy, which is the conventional treatment option.
- Hospital Pharmacies at the Forefront of Adoption
As a result of patients being more knowledgeable about the many treatment choices available for TNBC, hospital pharmacies are expected to account for 70% of the market share in 2024. By virtue of their accessibility within institutional settings, hospital pharmacies are preferred by most of patients. Furthermore, they are backed by government money to ensure that drug prices are cheap. Because they are open around the clock and offer complete access to all pharmaceuticals, hospital pharmacies have a significant portion of the market share in the distribution channel sector.
Regional Analysis
North America Captures Nearly 70% Market Value Share
North America, which accounted for approximately 70% of the global triple negative breast cancer treatment market revenue in 2023, is expected to remain a dominant region throughout the anticipated period. Additionally, the regional market expansion can be attributed to the rise in breast cancer incidences in countries like the US, and Canada. The regional market demand will be expanded by the expansion of research activities in oncology. The primary revenue sources in the region are countries like the US, and Canada.
The Asia Pacific triple negative breast cancer treatment industry is expected to experience the highest CAGR in the coming years because of an increase in the number of breast cancer cases in countries such as China, South Korea, and Japan. The increase in the number of women in China, and the assistance provided by domestic NGO functions will facilitate the proliferation of regional market growth. Additionally, the industry in the region has experienced growth due to the utilization of traditional Chinese medicine to treat triple negative breast cancer.
Fairfield’s Competitive Landscape Analysis
The TNBC treatment market is highly competitive, with key players investing heavily in research and development to bring innovative therapies to the market. The competitive landscape is characterized by a robust pipeline of drugs, particularly in immunotherapy and targeted therapy segments. Collaborations and strategic partnerships are expected as companies seek to enhance their product portfolios and accelerate clinical development. Smaller biotech firms and start-ups also contribute to the dynamic market, often focusing on niche therapeutic approaches. The competition is further intensified by the ongoing patent expirations and the entry of generic alternatives, driving the need for continuous innovation and differentiation.
Key Market Companies
- AstraZeneca PLC
- Pfizer, Inc.
- F. Hoffman - La Roche Ltd.
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- Mylan N.V.
- Eli Lilly and Company
- Celgene Corporation
- Sanofi S.A.
- Seattle Genetics and Genentech
- Johnson & Johnson Services, Inc.
- Teva Pharmaceuticals Industries Ltd
- Sun Pharmaceuticals Industries Ltd
- Fresenius Kabi AG
Recent Industry Developments
- In February 2024, a pre-clinical study by the University of Adelaide demonstrated that CDDD11-8 can effectively inhibit the growth of triple negative breast cancer without causing any toxic adverse effects.
- In December 2023, Anixa Biosciences, Inc. disclosed new and revised positive outcomes from its Phase 1 clinical trial for its breast cancer vaccine in December 2023.
- In May 2023, Shanghai Junshi Biosciences Co., Ltd disclosed in May 2023 that the National Medical Products Administration ("NMPA") had authorized the supplemental new drug application ("NDA") for the company's anti-PD-1 monoclonal antibody, toripalimab.
An Expert’s Eye
- Expert analysts view the TNBC treatment industry as a rapidly evolving and promising field with significant unmet medical needs.
- While TNBC represents a smaller subset of breast cancers, its aggressive nature and limited treatment options have driven intense research and development.
- The industry is characterized by substantial investment in novel therapies targeting specific molecular pathways involved in TNBC.
- While challenges persist in terms of treatment resistance and patient heterogeneity, the pipeline of emerging drugs and combination therapies offers hope for improved patient outcomes.
- The TNBC treatment landscape is poised for significant growth and transformation in the coming years.
Global Triple Negative Breast Cancer Treatment Market is Segmented as-
By Drug Type
- Doxorubicin
- Cyclophosphamide
- Paclitaxel
- Docetaxel
- Others
By Distribution Channel
- Hospital Pharmacies
- Specialty Cancer Clinics
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
1. Executive Summary
1.1. Global Triple Negative Breast Cancer Treatment Market Snapshot
1.2. Future Projections
1.3. Key Market Trends
1.4. Regional Snapshot, by Value, 2024
1.5. Analyst Recommendations
2. Market Overview
2.1. Market Definitions and Segmentations
2.2. Market Dynamics
2.2.1. Drivers
2.2.2. Restraints
2.2.3. Market Opportunities
2.3. Value Chain Analysis
2.4. Porter’s Five Forces Analysis
2.5. COVID-19 Impact Analysis
2.5.1. Supply
2.5.2. Demand
2.6. Impact of Ukraine-Russia Conflict
2.7. Economic Overview
2.7.1. World Economic Projections
2.8. PESTLE Analysis
3. Global Triple Negative Breast Cancer Treatment Market Outlook, 2019 - 2031
3.1. Global Triple Negative Breast Cancer Treatment Market Outlook, by Drug Type, Value (US$ Mn), 2019 - 2031
3.1.1. Key Highlights
3.1.1.1. Doxorubicin
3.1.1.2. Cyclophosphamide
3.1.1.3. Paclitaxel
3.1.1.4. Docetaxel
3.1.1.5. Carboplatin/Cisplatin
3.1.1.6. Others
3.2. Global Triple Negative Breast Cancer Treatment Market Outlook, by Distribution Channel, Value (US$ Mn), 2019 - 2031
3.2.1. Key Highlights
3.2.1.1. Hospital Pharmacies
3.2.1.2. Specialty Cancer Clinics
3.3. Global Triple Negative Breast Cancer Treatment Market Outlook, by Region, Value (US$ Mn), 2019 - 2031
3.3.1. Key Highlights
3.3.1.1. North America
3.3.1.2. Europe
3.3.1.3. Asia Pacific
3.3.1.4. Latin America
3.3.1.5. Middle East & Africa
4. North America Triple Negative Breast Cancer Treatment Market Outlook, 2019 - 2031
4.1. North America Triple Negative Breast Cancer Treatment Market Outlook, by Drug Type, Value (US$ Mn), 2019 - 2031
4.1.1. Key Highlights
4.1.1.1. Doxorubicin
4.1.1.2. Cyclophosphamide
4.1.1.3. Paclitaxel
4.1.1.4. Docetaxel
4.1.1.5. Carboplatin/Cisplatin
4.1.1.6. Others
4.2. North America Triple Negative Breast Cancer Treatment Market Outlook, by Distribution Channel, Value (US$ Mn), 2019 - 2031
4.2.1. Key Highlights
4.2.1.1. Hospital Pharmacies
4.2.1.2. Specialty Cancer Clinics
4.3. North America Triple Negative Breast Cancer Treatment Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
4.3.1. Key Highlights
4.3.1.1. U.S. Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
4.3.1.2. U.S. Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
4.3.1.3. Canada Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
4.3.1.4. Canada Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
4.3.2. BPS Analysis/Market Attractiveness Analysis
5. Europe Triple Negative Breast Cancer Treatment Market Outlook, 2019 - 2031
5.1. Europe Triple Negative Breast Cancer Treatment Market Outlook, by Drug Type, Value (US$ Mn), 2019 - 2031
5.1.1. Key Highlights
5.1.1.1. Doxorubicin
5.1.1.2. Cyclophosphamide
5.1.1.3. Paclitaxel
5.1.1.4. Docetaxel
5.1.1.5. Carboplatin/Cisplatin
5.1.1.6. Others
5.2. Europe Triple Negative Breast Cancer Treatment Market Outlook, by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.2.1. Key Highlights
5.2.1.1. Hospital Pharmacies
5.2.1.2. Specialty Cancer Clinics
5.3. Europe Triple Negative Breast Cancer Treatment Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
5.3.1. Key Highlights
5.3.1.1. Germany Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.2. Germany Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.1.3. U.K. Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.4. U.K. Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.1.5. France Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.6. France Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.1.7. Italy Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.8. Italy Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.1.9. Turkey Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.10. Turkey Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.1.11. Russia Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.12. Russia Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.1.13. Rest of Europe Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
5.3.1.14. Rest of Europe Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
5.3.2. BPS Analysis/Market Attractiveness Analysis
6. Asia Pacific Triple Negative Breast Cancer Treatment Market Outlook, 2019 - 2031
6.1. Asia Pacific Triple Negative Breast Cancer Treatment Market Outlook, by Drug Type, Value (US$ Mn), 2019 - 2031
6.1.1. Key Highlights
6.1.1.1. Doxorubicin
6.1.1.2. Cyclophosphamide
6.1.1.3. Paclitaxel
6.1.1.4. Docetaxel
6.1.1.5. Carboplatin/Cisplatin
6.1.1.6. Others
6.2. Asia Pacific Triple Negative Breast Cancer Treatment Market Outlook, by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.2.1. Key Highlights
6.2.1.1. Hospital Pharmacies
6.2.1.2. Specialty Cancer Clinics
6.3. Asia Pacific Triple Negative Breast Cancer Treatment Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
6.3.1. Key Highlights
6.3.1.1. China Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
6.3.1.2. China Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.3.1.3. Japan Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
6.3.1.4. Japan Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.3.1.5. South Korea Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
6.3.1.6. South Korea Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.3.1.7. India Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
6.3.1.8. India Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.3.1.9. Southeast Asia Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
6.3.1.10. Southeast Asia Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.3.1.11. Rest of Asia Pacific Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
6.3.1.12. Rest of Asia Pacific Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
6.3.2. BPS Analysis/Market Attractiveness Analysis
7. Latin America Triple Negative Breast Cancer Treatment Market Outlook, 2019 - 2031
7.1. Latin America Triple Negative Breast Cancer Treatment Market Outlook, by Drug Type, Value (US$ Mn), 2019 - 2031
7.1.1. Key Highlights
7.1.1.1. Doxorubicin
7.1.1.2. Cyclophosphamide
7.1.1.3. Paclitaxel
7.1.1.4. Docetaxel
7.1.1.5. Carboplatin/Cisplatin
7.1.1.6. Others
7.2. Latin America Triple Negative Breast Cancer Treatment Market Outlook, by Distribution Channel, Value (US$ Mn), 2019 - 2031
7.2.1. Key Highlights
7.2.1.1. Hospital Pharmacies
7.2.1.2. Specialty Cancer Clinics
7.3. Latin America Triple Negative Breast Cancer Treatment Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
7.3.1. Key Highlights
7.3.1.1. Brazil Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
7.3.1.2. Brazil Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
7.3.1.3. Mexico Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
7.3.1.4. Mexico Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
7.3.1.5. Argentina Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
7.3.1.6. Argentina Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
7.3.1.7. Rest of Latin America Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
7.3.1.8. Rest of Latin America Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
7.3.2. BPS Analysis/Market Attractiveness Analysis
8. Middle East & Africa Triple Negative Breast Cancer Treatment Market Outlook, 2019 - 2031
8.1. Middle East & Africa Triple Negative Breast Cancer Treatment Market Outlook, by Drug Type, Value (US$ Mn), 2019 - 2031
8.1.1. Key Highlights
8.1.1.1. Doxorubicin
8.1.1.2. Cyclophosphamide
8.1.1.3. Paclitaxel
8.1.1.4. Docetaxel
8.1.1.5. Carboplatin/Cisplatin
8.1.1.6. Others
8.2. Middle East & Africa Triple Negative Breast Cancer Treatment Market Outlook, by Distribution Channel, Value (US$ Mn), 2019 - 2031
8.2.1. Key Highlights
8.2.1.1. Hospital Pharmacies
8.2.1.2. Specialty Cancer Clinics
8.3. Middle East & Africa Triple Negative Breast Cancer Treatment Market Outlook, by Country, Value (US$ Mn), 2019 - 2031
8.3.1. Key Highlights
8.3.1.1. GCC Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
8.3.1.2. GCC Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
8.3.1.3. South Africa Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
8.3.1.4. South Africa Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
8.3.1.5. Egypt Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
8.3.1.6. Egypt Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
8.3.1.7. Nigeria Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
8.3.1.8. Nigeria Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
8.3.1.9. Nigeria Triple Negative Breast Cancer Treatment Market Distribution Channel, Value (US$ Mn), 2019 - 2031
8.3.1.10. Rest of Middle East & Africa Triple Negative Breast Cancer Treatment Market by Drug Type, Value (US$ Mn), 2019 - 2031
8.3.1.11. Rest of Middle East & Africa Triple Negative Breast Cancer Treatment Market by Distribution Channel, Value (US$ Mn), 2019 - 2031
8.3.2. BPS Analysis/Market Attractiveness Analysis
9. Competitive Landscape
9.1. Drug Type vs Distribution Channel Heatmap
9.2. Manufacturer vs Distribution Channel Heatmap
9.3. Company Market Share Analysis, 2022
9.4. Competitive Dashboard
9.5. Company Profiles
9.5.1. AstraZeneca PLC
9.5.1.1. Company Overview
9.5.1.2. Drug Type Portfolio
9.5.1.3. Financial Overview
9.5.1.4. Business Strategies and Development
9.5.2. Pfizer, Inc.
9.5.2.1. Company Overview
9.5.2.2. Drug Type Portfolio
9.5.2.3. Financial Overview
9.5.2.4. Business Strategies and Development
9.5.3. F. Hoffman - La Roche Ltd.
9.5.3.1. Company Overview
9.5.3.2. Drug Type Portfolio
9.5.3.3. Financial Overview
9.5.3.4. Business Strategies and Development
9.5.4. Bristol-Myers Squibb Company
9.5.4.1. Company Overview
9.5.4.2. Drug Type Portfolio
9.5.4.3. Financial Overview
9.5.4.4. Business Strategies and Development
9.5.5. Mylan N.V.
9.5.5.1. Company Overview
9.5.5.2. Drug Type Portfolio
9.5.5.3. Financial Overview
9.5.5.4. Business Strategies and Development
9.5.6. Celgene Corporation
9.5.6.1. Company Overview
9.5.6.2. Drug Type Portfolio
9.5.6.3. Financial Overview
9.5.6.4. Business Strategies and Development
9.5.7. Sanofi S.A.
9.5.7.1. Company Overview
9.5.7.2. Drug Type Portfolio
9.5.7.3. Financial Overview
9.5.7.4. Business Strategies and Development
9.5.8. Seattle Genetics and Genentech
9.5.8.1. Company Overview
9.5.8.2. Drug Type Portfolio
9.5.8.3. Financial Overview
9.5.8.4. Business Strategies and Development
9.5.9. Johnson & Johnson Services, Inc.
9.5.9.1. Company Overview
9.5.9.2. Drug Type Portfolio
9.5.9.3. Financial Overview
9.5.9.4. Business Strategies and Development
9.5.10. Teva Pharmaceuticals Industries Ltd
9.5.10.1. Company Overview
9.5.10.2. Drug Type Portfolio
9.5.10.3. Financial Overview
9.5.10.4. Business Strategies and Development
9.5.11. Sun Pharmaceuticals Industries Ltd
9.5.11.1. Company Overview
9.5.11.2. Drug Type Portfolio
9.5.11.3. Financial Overview
9.5.11.4. Business Strategies and Development
9.5.12. Fresenius Kabi AG
9.5.12.1. Company Overview
9.5.12.2. Drug Type Portfolio
9.5.12.3. Financial Overview
9.5.12.4. Business Strategies and Development
10. Appendix
10.1. Research Methodology
10.2. Report Assumptions
10.3. Acronyms and Abbreviations
|
BASE YEAR |
HISTORICAL DATA |
FORECAST PERIOD |
UNITS |
|||
|
2023 |
|
2019 - 2023 |
2024 - 2031 |
Value: US$ Million |
||
|
REPORT FEATURES |
DETAILS |
|
Drug Type Coverage |
|
|
Distribution Channel Coverage |
|
|
Geographical Coverage |
|
|
Leading Companies |
|
|
Report Highlights |
Key Market Indicators, Macro-micro economic impact analysis, Technological Roadmap, Key Trends, Driver, Restraints, and Future Opportunities & Revenue Pockets, Porter’s 5 Forces Analysis, Historical Trend (2019-2021), Market Estimates and Forecast, Market Dynamics, Industry Trends, Competition Landscape, Category, Region, Country-wise Trends & Analysis, COVID-19 Impact Analysis (Demand and Supply Chain) |
